
Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Multiple bonds are also beneficial for understanding nuclear magnetic resonance spectra (NMR). Multiple bonds influence a molecule's electrical characteristics and can change its physical properties such as the boiling point and melting point. A double bond has one sigma and one pi bond, while a triple bond has one sigma and two pi bonds. In single bonds only a sigma bond is present, but multiple bonds have both sigma and pi bonds. Multiple bonds are seen in covalent compounds (double or triple bonds). Shape of a molecule is not determined by the Pi bond. Shape of a molecule is determined by the sigma bond. Sigma bond has cylindrical charge symmetry around the bond axis. There are two pi bonds in the triple bond. There is one sigma bond in a triple bond. There is only one pi bond observed in a double bond. There is one sigma bond in a double bond. Pi bonds are not involved in the control of geometry in polyatomic molecules. Sigma bonds are involved in the control of geometry in polyatomic molecules. There can be two pi bonds between two atoms.Ĭontrol of Geometry in Polyatomic Molecules There is only one sigma bond between two atoms. Pi bonds are formed after the formation of sigma bonds. Sigma bonds are formed first when atoms come closer.
Pi bonds are less strong than sigma bonds. The strength of sigma bonds is more than pi bonds. Pi-bond always exists along with sigma bonds. In sigma bonds, orbitals may overlap: two hybrid orbitals, one hybrid and one pure orbital or two pure orbitals.įor pi bonds, two pure (i.e., unhybridised) orbitals are always alternating orbitals. Pi bonds are formed through the lateral overlap of the half - filled atomic orbitals. Sigma bonds are formed by the axial overlap of half- filled atomic orbitals. Further, it is important to note that pi bond(s) are produced in addition to a sigma bond in the formation of multiple bonds between two atoms of a molecule.ĭifference Between Sigma Bond and Pi Bond Therefore, it is stronger than the pi bond, where the extent of overlap occurs to a lesser extent. The duplication of orbitals arises to a greater degree in the case of a sigma bond. The side-overlapping orbitals consist of two types of saucer-charged clouds above and below the surface of the atoms involved.Įssentially, a bond's strength depends on the extent to which it overlaps. Throughout pi-bond formation, the atomic orbitals converge so that their axes appear parallel to each other and perpendicular to the central axis. The axes of the atomic orbitals are parallel to one other during bond formation, whereas the overlapping is perpendicular to the internuclear axis. Pi bonds are formed when atomic orbitals intersect in a sideways positive (same phase) direction perpendicular to the internuclear axis. This sort of overlap exists between half-filled p-orbitals of the two atoms that approach. This sort of overlap takes place between half-full s-orbitals of one atom and half-full p-orbitals of another. In this case, two half-filled s-orbitals are interacting along the internuclear axis, as shown below. Any of the following types of combinations of atomic orbitals may form this. This is called head overlapping or axial overlapping. This type of covalent bond is formed by the overlap of bonding orbitals along the internuclear axis from end to end (head-on). For example, the methane molecule contains 4 C-H sigma bonds. The covalent bond formed by the axial overlap of atomic orbitals is called a sigma bond. Both are used extensively to predict the behaviour of molecules in molecular orbital theory. Generally, sigma bonds are stronger than pi bonds. This overlap happens in two ways, resulting in two different types of covalent bonds: sigma and pi bonds. Various bond properties, including bond length, bond angle, and bond enthalpy, are influenced by how atomic orbitals overlap.
Both names, sigma and pi, are derived from the Greek letters.

The head-to-head overlapping of atomic orbitals forms sigma bonds, whereas the lateral overlap of two atomic orbitals forms pi bonds. The overlapping of atomic orbitals forms covalent bonds.
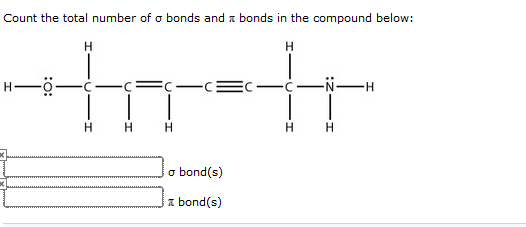
The overlapping of atomic orbitals distinguishes sigma and pi bonds from each other.


 0 kommentar(er)
0 kommentar(er)
